We can thank (or curse) our ancestors for the ability of our bodies to digest alcohol. Over millions of years, our ancestors adapted to the presence of rotted and fermented organic material by preferencing genetic lines that were able to break down Ethanol using an enzyme called ADH, first into a toxic component (Acetaldehyde), and then quickly changing the toxin into Acetate using an enzyme called ALDH for excretion.
Those hangovers you experienced, the throwing up, the nausea, … that means you drank too much, too fast, and your ALDH process could not keep up with your ADH process, and Acetaldehyde built up in your system poisoning you.
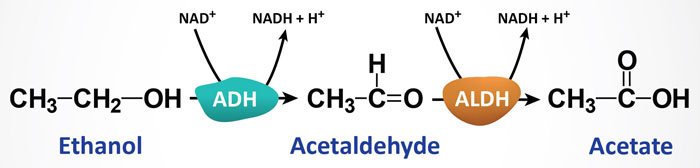
Pathways of Ethanol Metabolism
Ethanol is a small two carbon alcohol that, due to its small size and alcoholic hydroxyl group (OH) it is polar (due to double negative = O and positive + H) and hydrophilic and becomes soluble in both aqueous and lipid environments. This allows ethanol to freely pass from bodily fluids into cells. Since the portal circulation from the small intestines passes first through the liver, the bulk of ingested alcohol is metabolized in the liver. The process of ethanol oxidation involves at least three distinct enzymatic pathways.
The most significant pathway, responsible for the bulk of ethanol metabolism, is that initiated by alcohol dehydrogenase, ADH. As outlined below, humans express several ADH genes with the class I members being responsible for hepatic ethanol metabolism. The ADH enzymes are NAD+-requiring and they are expressed at high concentrations in hepatocytes. Animal cells (primarily hepatocytes) contain cytosolic ADH which oxidizes ethanol to acetaldehyde. Acetaldehyde then enters the mitochondria where it is oxidized to acetate by mitochondrial aldehyde dehydrogenase (ALDH). A cytosolic ALDH exists but is responsible for only a minor amount of acetaldehyde oxidation.
Alcohol and the individual
Ingestion
Absorption through the stomach and intestines
When an alcoholic beverage is swallowed, it is diluted by gastric juices in the stomach. A small portion of the alcohol is diffused into the bloodstream directly from the stomach wall, but most passes through the pyloric junction into the small intestine, where it is very rapidly absorbed. However, up to half the alcohol is degraded in the stomach before it passes into the small intestine. In general, a lower percentage of the alcohol is degraded in a young woman’s stomach than in a young man’s because a young woman’s gastric secretions contain lower levels of the enzyme alcohol dehydrogenase (ADH), which breaks down alcohol prior to absorption.
ethanol, also called ethyl alcohol, grain alcohol, or alcohol, a member of a class of organic compounds that are given the general name alcohols; its molecular formula is C2H5OH. Ethanol is an important industrial chemical; it is used as a solvent, in the synthesis of other organic chemicals, and as an additive to automotive gasoline (forming a mixture known as a gasohol). Ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits.
Ethanol is converted in the body first to acetaldehyde and then to carbon dioxide and water, at the rate of about half a fluid ounce, or 15 ml, per hour; this quantity corresponds to a dietary intake of about 100 calories.
acetaldehyde (CH3CHO), also called ethanal, an aldehyde used as a starting material in the synthesis of 1-butanol (n–butyl alcohol), ethyl acetate, perfumes, flavourings, aniline dyes, plastics, synthetic rubber, and other chemical compounds. It has been manufactured by the hydration of acetylene and by the oxidation of ethanol (ethyl alcohol). Today the dominant process for the manufacture of acetaldehyde is the Wacker process, developed between 1957 and 1959, which catalyzes the oxidation of ethylene to acetaldehyde. The catalyst is a two-component system consisting of palladium chloride, PdCl2, and copper chloride, CuCl2.

Pure acetaldehyde is a colourless, flammable liquid with a pungent, fruity odour; it boils at 20.8 °C (69.4 °F).
Leave a comment